INTRODUCTIONThe (ATL) American Tegumentary Leishmaniasis is an infectious, chronic, non-contagious disease (1,2,3), caused by
Leishmania gender protozoa, and the main species are
Leishmania (Viannia) braziliensis, Leishmania (Viannia) guyanensis and
Leishmania (Leishmania) amazonensis (4). It is primarily a zoonotic infection of wild animals and more rarely domestic animals, including marsupial, carnivore and even primates, and man is an accidental host (2,5).
All species of
Leishmania are transmitted by the shot hole of female mosquitoes called phlebotomines, which belong to the
Lutzomyia and
Phlebotomus genders, and this transmission is made by inoculation of the promastigot forms in the skin of the vertebrate host (2,3,4,5).
The ATL occurs in the Americas from the South of the United States up to the north of Argentina. The most important focus is the South-American one, which comprises all of its countries, except for Uruguay and Chile (5).
In 1999, 30,550 ATL autochthon cases were identified in Brazil, and the detection coefficient is of 18.63/100.000 inhabitants (2). In 2003, the regions with major predominance of ATL were North (14200 cases) and Northeast (8005 cases) (3). Initially, the transmitter mosquito reservoirs were sylvestral or in agricultural areas, but the environmental transformations, provoked by the migratory process and growing urbanization have been modifying this profile. The vectors adaptation to new conditions have enabled the disease diffusion in the domiciliary and peridomiciliary coverage (1,4).
The cutaneous tissue and the mucosa are the most commonly affected and the most common manifestation is the leishmaniotic ulcer: sole cutaneous ulcer or in low number, with elevated borders, in frame and with absence of local pain. Other morphologic aspects may also be identified, such as: infiltrated plate, tubercle, node and vegetating verrucous lesion. When the mucosa is injured, it may have an infiltrated erythema, granulose or ulcerated aspect. In order of frequency, the mucous lesions manifest mainly in the nose, hard palate, pharynx and larynx (1,4).
Not only in Brazil, but also in other countries of the New World, ATL is a Public Health problem. Its importance is not only on its high incidence and large geographic distribution, but also in the possibility to present forms that may determine destructive, disfigurating and also incapacitating lesions, with great repercussion in the individual's psychosocial field.
HistoryHistorical statements suggest that ATL affected peoples of America even before the contact with Europeans and Africans. It is thought to have been originated in the western Amazon area in archeological ages by means of human migrations, then spread to the high jungle and after to interandean hot lands, at the limits of Bolivia and Pery with Brazil (6).
In Brazil, the cutaneous and nasopharyngeal legions leishmaniotic nature was only confirmed for the first time in 1909, by Lindenberg, who found leishmania forms identical to the agent of the
Leishmania tropica (of the Old World), in cutaneous lesions of individuals who worked in the forest of São Paulo countryside (1,3).
In 1911, Gaspar Vianna gave the parasite, found by Lindenberg, the name of
Leishmania brasiliensis, for having deemed it to be morphologically different from the
Leishmania tropica. And characterized, from then on, the disease etiologic agent referred to as "úlcera de Bauru" [Bauru's ulcer], "ferida brava" [wild injury] or "nariz de anta" [tapir's nose] (1,3).
In many studies in the decades of thirties and forties they verified in the Santa Casa de São Paulo that almost all the mucous leishmaniasis lesions had their origin in the nasal mucosa (7,8,9). In this place, we can observe sometimes in the septum the presence of clearly defined polyps, similar, under the clinical viewpoint, to the common polyps, a lesion described for the first time in 1925 and referred to as "leishmaniasis polyp" (10).
Along the years, the lesions were far better defined, including microscopically, mainly due to the advent of diagnostic trials, as smear, culturally and histopathologically (11).
ATL was initially treated by Gaspar Vianna with emetic tartar, that a few decades after was replaced by antimony pentavalents as the choice drugs (1,12). Glucanthyme, the principal group drug, was only availed in Brazil after the Second World War and is used until today.
StadiamentThe ATL is a initial infection of the skin (its preferential location site) from which it can undergo propagation of a secondary process that manifest in the upper respiratory passages mucosa.
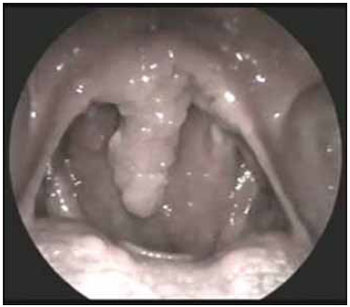
Picture 1. Leishmaniasis: Uvula commitment.
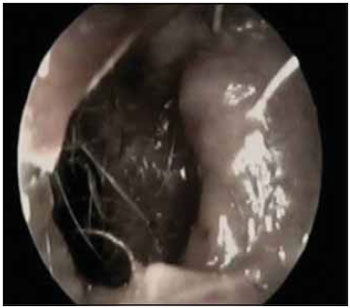
Picture 2. Leishmaniasis: Destruction of the nasal septum, seen by the left nasal cavity.
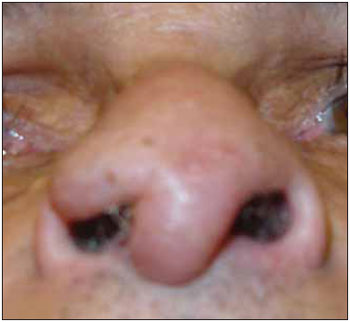
Picture 3. Leishmaniasis: Deformity of the nasal wing.
Basically it is possible to make the stadiament of the lesions occurred in the ATL by taking into account the lesion time of appearing, extension and dissemination and group them into:
1. Primary infections: that characterizes the primary accident (initial lesion) or leishmaniotic cancer, found in the shot hole place, and after the incubation period (from two weeks to one year) there appear erythematous papules that progress up to the formation of ulcers with serobloody crust (11,14).
2. The secondary leishmaniasis appear in a variable basis from one to three months after the primary infection, affecting the skin, ganglions, lymphatic organs and mucosa, and by contiguity the mucosa of nose, lips, eyelids and genitals are committed when the primary or secondary lesions are installed close to theses regions (11,14).
3. The tertiary leishmaniasis needs a longer period to appear and it happens generally five to ten years after the initial lesion and is characterized by the presence of naso-buco-pharyngeal, laryngeal and ocular lesions, and in this tertiary period the primary infection has already disappeared and the secondary infection may, in general, still be present (11,14).
Clinical manifestationsWe must remark first that the mucosa leishmaniasis manifests almost always after the cutaneous affection, no matter the variation of the latter and its location point (14). The mucous affection may arouse with the cutaneous lesion still active, or years after healing, and such time is very variable, according to the patient immunologic predisposition (1,5,13).
In order of frequency, the mucous lesions manifest, mainly, in the nose, hard palate, pharynx and larynx (1,2,8,13,15,17), where they can be present with an infiltrated erythema, granulose, ulcerated or polypoid aspect with a surface roughly rounded (1,5,16). The death of the patient generally occurs for aspiration or respiratory insufficiency (15).
Clinical manifestations in the otorhinolaryngology
Nasal CavityIt is the place preferably affected in almost all the leishmaniotic mucous lesions (1,2,7,8,9,11,13,14,17).
Some hypothesis try to clarify the reason why of such a preference. The direct contact is supposed, that is, the individual touches the primary cutaneous lesion and then scratches the nose, and disseminates it to the mucosa, or by the skin cutaneous lesions contiguity. However too few cases have been reported with this type of transmission (2,13).
Another hypothesis is that the tissue elements of the frontal part of the nasal cavities offer optimum conditions to the Leishmanias location. The transition zone between the pavimentous epithelium and the pseudo-stratified vibratile element, in the front portion of the nasal septum and the head of the inferior contour, form the "locus minoris resistentia" to the leishmaniotic process (7).
However, the most consistent hypothesis states that the Leishmania needs lower temperatures for its growth. Thus, since the front area of the nasal septum is more cooled due to the inspiration air current, there would be preference for the proliferation of the parasites (13).
The nasal cartilage specific destruction may also indicate autoimmune reaction, which would explain the reason why some patients present intense tissue destruction while others only have mucous affection some decades later. The antibodies search was also suggested against the Type II collagen, similar to those of the rheumatoid arthritis (13).
Upon physical exam we observe mucosa circumscribed hyperemia and slight infiltration. Then the ulcerative process begins (ulcerovegetating/polypoid, ulcerocrustous or ulcerodestructive lesions), which provokes septal and sometimes subseptal perforation resulting in the dropping of the nasal tip (1,2,11,16,18).
The lesion goes on with ulceration in the nose wings and subsequent generalized nasal infiltration or destruction (in mucosa and cartilages), and exposes the subjacent structures (1,18). The osseous implication is not frequent, but there are reports of the infundibula secondary osteomyelitis, that determines membrane ulceration aspect (11,13).
The nasal pyramid skin is many times with erythema, edema and hypertrophy and even recalls potato nose. There may occur partial or total destruction of this region (2,11,14).
This lesion process results in a frontal nose collapse, with enlargement and flattening, which is referred to as "Tapir's nose". Upon raising the nose tip, sometimes all the internal structure seem to have been destroyed and the rear wall of the nasopharynx is visible (13).
The main signals and symptoms include nasal obstruction, recurrent epistaxis associated or not with sneezing crises, burning and/or pain at forced breathing, rhinorhea, crusts formation and even elimination of necrosed tissue (1,2,11,13,14,16,18). The appearing of secondary infection is frequent provoking pain, cacosmia and bloody rhinorhea. We can also verify secondary folliculitis in the nasal vestibule (11,14).
In a study carried out with 41 patients with nasal Leishmaniasis diagnosis in 2006, we confirmed as the most frequent symptoms obstruction (75%), epistaxis (48%) and rhinorhea (39%). The nasal pyramid was normal in 24% with the tip dropped in 19% and enlarged in 14%. The main aspects of the nasal mucosa were: presence of crusts (83%) granulose (61%) infiltrated and edematous (22%) (19).
In a report of four cases o ATL associated with HIV virus serviced in the Hospital Universitário de Brasília, ulceration in nasal mucosa associated with rhinorhea, nasal obstruction and epistaxis one year ago was verified: upon physical examination: infiltration, erythema on the dorsum of nose and ulcer in nasal mucosa with partial destruction of the septum. infiltrating lesions and edema on dorsum of nose were also confirmed. It is important to observe the severity of the lesions associated with the immunosuppression of the relevant patients (20).
Other study reports a patient serviced at HC-FMUSP with complaint of ulcerated lesion in the nasal and upper lip region three years ago. upon physical examination: perforation of nasal septum, exposing hyperemiad quadrangular cartilage borders and with granulomatous aspect, evolving after one year with destruction of the nasal pyramid inferior wall, the infundibula and lateral wall of the nose, with exposure of the maxillary sinus.
Palate, Pharynx and LarynxThe oral cavity, pharynx and larynx lesions rarely occur isolated and they are more frequently associated with initial lesion of the nasal mucosa (7,13,21).
In the mouth, the hard palate is frequently committed with dissemination of the process for the soft palate, uvula and pharynx. The proliferating infiltrating process may cause fusion of the uvula, pillars, lateral strings and posterior wall, originating a nasopharynx obliteration. Deformity and narrowing of the oropharynx light may occur due to fibrosis of the amygdaline cavity (13,14).
The palatine veil infiltration reaches proportions of a real tumor mass. All the palate appears changed: the uvula is reduced to an unshapely mass of uneven vegetating surface. In the roof of the mouth lobate prominences are formed, being separated by sinuous sulcus and ulcerated erosions (13,14).
More rarely it can include the gingival and dental interstices, where lengthy and prominent granulations are developed reaching to the upper lip. The tongue is usually preserved (13,14).
The hypopharynx, larynx, epiglottis, arytenoids cartilages and posterior commissures of the vocal cords are covered by lesion of vegetating aspect and sometimes they end up uniting. Such granulations frequently regress and end up disappearing, and the affected surface takes a plain aspect and coloration slightly whitish.
There is laryngeal generalized inflammation particularly in the region of the pear-shaped sinuses. The vocal cords seem to be moving well, but the phonation is weak and the muscular control of the tension may be damaged by granulomatous formation and subsequent fibrosis. Even after the treatment success, rarely the voice returns to the normal pattern and the larynx light may be reduced (13). The phonoaudiologist actuation is crucial.
As the most frequent complaints, the following are remarkable: Mouth injuries and sialorrhoea, odinophagy due to the pharyngeal affections, cough, dysphonia or even aphonia because of laryngeal commitment. The complications include pneumonias for aspiration, bacterial infections, secondary myiasis, cachexia for difficulties in the deglutition, edema of glottis and asphyxia, which can be followed by the death of the patient mainly due to the respiratory insufficiency and sepsis (1,2,13,14,16).
In a report of pseudo-haemoptysis for leishmaniasis, the patient started a case of cough, soft and sporadic haemoptysis, anorexia, loss of weight, night sudoresis, apathy and intermittent raucousness for 15 months, evolving with odinophagy, dysphagy and loss of 16kg in 6 months. Upon oroscopy: bad dentition, vegetating, nodular, verrucous, ulcerated, erythematic lesions committing the soft palate, uvula and posterior wall of the pharynx. In the video-laryngo-bronchoscopy he presented vegetating, nodular, verrucous, ulcerated, friable lesions with easy bleeding at the touch of the appliance, which expanded from the soft palate to the larynx. Bilaterally committed vocal cords, but with mobility preserved (21).
Another report of patient with palate leishmaiose, co-infected with HIV, presented granulomatous and ulcerated lesions in the soft palate. The video-laryngoscopy confirmed an involvement of the larynx and epiglottis, with infiltration of the vestibular cords and arytenoid cartilages and moderate reduction of the respiratory passage calibration (22).
A last case reports a child of 5 years of age with nasal leishmaniasis treated nonspecifically as allergic rhinitis which complicated with perforation of the nasal septum, destruction of the alar cartilage, granulose lesion in the hard palate, lesions infiltrated in the chin and left malar region and cervical polyadenomegaly. It evolved with fall of the general state and severing of the lesions, reaching nose, lips, hard and soft palate, left perioral and malar regions with necrosis and extensive destruction. It was finally followed by bronchopneumonia and sepsis, which led the patient to death (23).
EarThe skin and auricular cartilage affection occurs for they are a location of lower temperature, subject to the leishmania growth, besides being an area exposed to inoculation of vectors (24).
The external ear commonly presents an increase of volume, ulcers with elevated borders, sometimes covered by crusts, and may appear as an infiltrated plate, tubercle, node and verrucous vegetating lesion, leading finally to mutilation of the auricular pavilion (1,2,16,24).
CONCLUSIONThe knowledge regarding tropical endemic diseases relating to the upper respiratory passages and the understanding of their relations with the otorhinolaryngology are extremely important for the resolution of such lesions, as well as to prevent the deformities caused to the affected structures.
BIBLIOGRAPHIC REFERENCES1. Silveira FT, Lainson R, Brito AC, Oliveira MRF, Paes MG, Souza AAA, e col. Leishmaniose tegumentar americana. In: Leão, RNQ. Doenças infecciosas e parasitárias enfoque amazônico. 1ª ed. Belém: Cejup/ UEPA/ Instituto Evandro Chagas; 1997, p.619-30.
2. Ministério da Saúde. Fundação Nacional de Saúde. Manual de Controle da Leishmaniose Tegumentar Americana. 2000. Disponível em: http://portal.saude.gov.br/portal/arquivos/pdf/manu_leishman.pdf. Acessado em: 19 de março de 2007.
3. Basano AS, Camargo LMA. Leishmaniose tegumentar americana: histórico, epidemiologia e perspectivas de controle. Rev Bras Epidemiol. 2004, 7(3):328-37. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext& pid=S1415-790X2004000300010&lng=en&nrm=iso. Acessado em: 19 de março de 2007.
4. Ministério da Saúde. Fundação do Instituto Oswaldo Cruz. Instituto de Pesquisa Clínica Evandro Chagas. Leishmanioses. 2006. Disponível em: http://www.ipec.fiocruz.br/pepes/leish/leish.html. Acessado em 19 de março de 2007.
5. Gontijo B, Carvalho MLR. Leishmaniose Tegumentar Americana. Rev Soc Bras Med Trop. 2003, 36(1):71-80. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0037-86822003000100011&lng=en&nrm=iso. Acessado em: 19 de março de 2007.
6. Altamirano-Enciso AJ, Marzochi MCA, Moreira JS, Schubach AO, Marzochi KBF. Sobre a origem e dispersão das leishmanioses cutânea e mucosa com base em fontes históricas pré e pós-colombianas. Hist. Cienc. Saude-Manguinhos. 2003, 10(3):853-82. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0104-59702003000300004&lng=pt&nrm=iso&tlng=pt. Acessado em: 19 de março de 2007.
7. Barbosa JER. Dados estatísticos sobre os casos de leishmaniose das mucosas observados no serviço de oto-rino-laringologia da Santa Casa de São Paulo. 1936, 4(5):697-714. Disponível em: http://www.rborl.org.br/conteudo/acervo/acervo.asp?id=1686. Acessado em: 19 de março de 2007.
8. Campos JA. Estatística dos casos de leishmaniose das mucosas, observados no serviço de oto-rino-laringologia da Santa Casa de São Paulo no lustro 1939-1943. Rev Bras Otorrinolaringol. 1944, 12(6):372-92. Disponível em: http://www.rborl.org.br/conteudo/acervo/acervo.asp?id=1362. Acessado em 19 de março de 2007.
9. Aquino FP. Estudo estatístico das formas mucosas da leishmaniose no serviço de oto-rinolaringologia da Santa Casa de Misericórdia de São Paulo, com considerações de ordem profilática. Rev Bras Otorrinolaringol. 1945, 13(2):105-21. Disponível em: http://www.rborl.org.br/conteudo/acervo/acervo.asp?id=680. Acessado em: 19 de março de 2007.
10. Falcão EC. O "pólipo da leishmaniose" (Em tôrno de uma nova observação desta forma clinica). Rev Bras Otorrinolaringol. 1935; 3(4):257-63. Disponível em: http://www.rborl.org.br/conteudo/acervo/acervo.asp?id=291. Acessado em: 19 de março de 2007.
11. Grellet M, Souza LCA. A Leishmaniose na Otorrinolaringologia. Rev Bras Otorrinolaringol. 1980, 46(2):142-9. Disponível em: http://www.rborl.org.br/conteudo/acervo/acervo.asp?id=1804. Acessado em: 19 de março de 2007.
12. Lindenberg A. Curabilidade e tratamento da leishmaniose ulcerosa e da blastomicose. Rev Bras Otorrinolaringol. 1936, 4(5):685-8. Disponível em: http://www.rborl.org.br/conteudo/acervo/acervo.asp?id=1684. Acessado em: 19 de março de 2007.
13. Marsden PD. Mucosal Leishmaniosis ("espúndia" Escomel, 1911). Trans. R. Soc. Trop. Med. Hyg. 1986, 80(6):859-76.
14- Barretto M. Das Formas Mucosas da Leishmaniose Tegumentar Americana e seu Tratamento. Rev Bras Otorrinolaringol. 1935, 3(5):446-61. Disponível em: http://www.rborl.org.br/conteudo/acervo/acervo.asp?id=300. Acessado em: 19 de março de 2007.
15. Amato VS, Oliveira LS, Silva ACM, Machado FR, Amato JGP, Nicodemo AC e col. Um caso de leishmaniose cutâneo-mucosa tratado com sucesso com baixa dose de antimonial pentavalente. Rev Soc Bras Med Trop. 1998, 31(2):221-4. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0037-86821998000200008&lng=pt&nrm=iso&tlng=pt. Acessado em: 24 de março de 2007.
16. Brito AC, Azulay DR, Azulay RD. Leishmaniose e demais protozooses de interesse dermatológico. In: Azulay RD, Azulay DR. Dermatologia. 4ª ed. Rio de Janeiro: Guanabara Koogan; 2006, p.396- 405.
17. Name RQ, Borges KT, Nogueira LSC, Sampaio JHD, Tauil PL, Sampaio RNR. Estudo clínico, epidemiológico e terapêutico de 402 pacientes com leishmaniose tegumentar americana atendidos no Hospital Universitário de Brasília, DF, Brasil. An Bras Dermatol. 2005, 80(3):249-254. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0365-05962005000300004&lng=pt&nrm=iso. Acessado em: 24 de março de 2007.
18. Fernandes NC, Morgan I, Maceira JP, Cuzzi T, Noe RAN. Leishmaniose tegumentar americana: casuística hospitalar no Rio de Janeiro. An Bras Dermatol. 2004, 79(4):431-9. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0365-05962004000400005&lng=pt&nrm=iso&tlng=pt. Acessado em: 24 de março de 2007.
19. Carvalho T, Dolci JEL. Avaliação clínica da influência do uso de glucantime em pacientes com Leishmaniose nasal. Acta ORL/Técnicas em Otorrinolaringologia. 2006, 24(2):77-82. Disponível em: http://www.actaorl.com.br/PDF/24-02-06.pdf, Acessado em: 24 de março de 2007.
20. Sampaio RNR, Salaro CP, Resende P, Paula CDR. Leishmaniose tegumentar americana associada à AIDS: relato de quatro casos. Rev Soc Bras Med Trop. 2002, 35(6):651-4. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0037-86822002000600017&lng=en&nrm=iso. Acessado em: 24 de março de 2007.
21. Meli SMD, Neto JCT, Andrade LCF. Pseudo-hemoptise por leishmaniose. J. Pneumologia. 1999, 25(6):347-50. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0102-35861999000600010&lng=pt&nrm=iso&tlng=pt. Acessado em: 24 de março de 2007.
22. Amato VS, Nicodemo AC, Amato JG, Boulos M, Neto VA. Mucocutaneous leishmaniasis associated with HIV infection treated successfully with liposomal amphotericin B (AmBisome). Journal of Antimicrobial Chemotherapy. 2000, 46(2):341-2. Disponível em: http://jac.oxfordjournals.org/cgi/content/full/46/2/341. Acessado em: 24 de março de 2007.
23. Velozo D, Cabral A, Ribeiro MCM, Motta JOC, Costa IMC, Sampaio RNR. Leishmaniose mucosa fatal em criança. An Bras Dermatol. 2006, 81(3):255-9. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0365-05962006000300008&lng=pt&nrm=iso. Acessado em: 24 de março de 2007.
24. Ecco R, Langohr IM, Schossler JEW, Barros SS, Barros CSL. Leishmaniose cutânea em cobaias (Cavia porcellus). Cienc Rural. 2000, 30(3):525-528. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-84782000000300027&lng=en&nrm=iso. Acessado em: 24 de março de 2007.
1. Master's Degree in Otorhinolaryngology by UFRJ and in Course for Doctor's Degree in Neuroscience by UFPA. (Assistant Professor of Otorhinolaryngology at UFPA and UEPA. Preceptor of the Medical Residence in Otorhinolaryngology of the Hospital Universitário Bettina Ferro de Souza of UFPA)
2. Student in the fourth year of the Medicine Course (Universidade do Estado do Pará)
3. Master's Degree in Otorhinolaryngology by UFRJ and in Course for Doctor's Degree in Neuroscience by UFPA. (Assistant Professor of Otorhinolaryngology of UEPA. Preceptor of the Medical Residence in Otorhinolaryngology of the Hospital Universitário Bettina Ferro de Souza of UFPA.)
4. Student in the sixth year of the Medicine Course (Universidade Federal do Pará.)
5. Student in the Second Year of the Medicine Graduation Course (Universidade do Estado do Pará)
Institution: (COP) Centro de Otorrinolaringologia do Pará. Belém / PA - Brazil.
Mail address:
Francisco Xavier Palheta Neto
Centro de Otorrinolaringologia do Pará
Avenida Conselheiro Furtado, 2391, sala 1608 - Bairro: Cremação
Belém / Pará - Zip code: 66040-100
E-mail: franciscopalheta@hotmail.com
Article received on March 31, 2008.
Article approved on July 31, 2008.